The emaciated girl’s stare. The lethargic boy’s protruding belly. The inert mother. From the sands of Egypt and Morocco to the rainforests of Brazil and Southeast Asia, Phil LoVerde, Ph.D., professor of biochemistry and pathology in the School of Medicine, has observed the human toll of schistosomiasis, the world’s most common parasitic disease after malaria.
In oases and villages, he has been a welcome visitor—a scientist sifting through clues like a detective, ever on a mission to end the menace and relieve the suffering.
“I’ve worked on ‘schisto’ my whole life,” Dr. LoVerde said. Indeed, searching for a better treatment has been his life’s work, and with the help of colleagues at Texas Biomedical Research Institute and The University of Texas at San Antonio, Dr. LoVerde is developing a new therapy to control the spread of schistosomiasis worldwide. A $3 million, five-year grant to the Health Science Center from the National Institute of Allergy & Infectious Diseases funds the studies.
Schistosomiasis, also called snail fever, is an infection of the larval worms of freshwater snails. These larvae, the size of a fleck of dust, infect 261 million people in 78 countries, according to the World Health Organization. Two-thirds of the world’s cases are in Africa.
[bgsection pex_attr_title=”” pex_attr_subtitle=”” pex_attr_undefined=”undefined” pex_attr_style=”section-custom” pex_attr_bgcolor=”” pex_attr_image=”” pex_attr_imageopacity=”1″ pex_attr_bgimagestyle=”static” pex_attr_titlecolor=”ffffff” pex_attr_textcolor=”a8230c” pex_attr_height=”500″]
Like mosquitoes transmit malaria, freshwater snails carry deadly larval worms that infect millions of people worldwide.
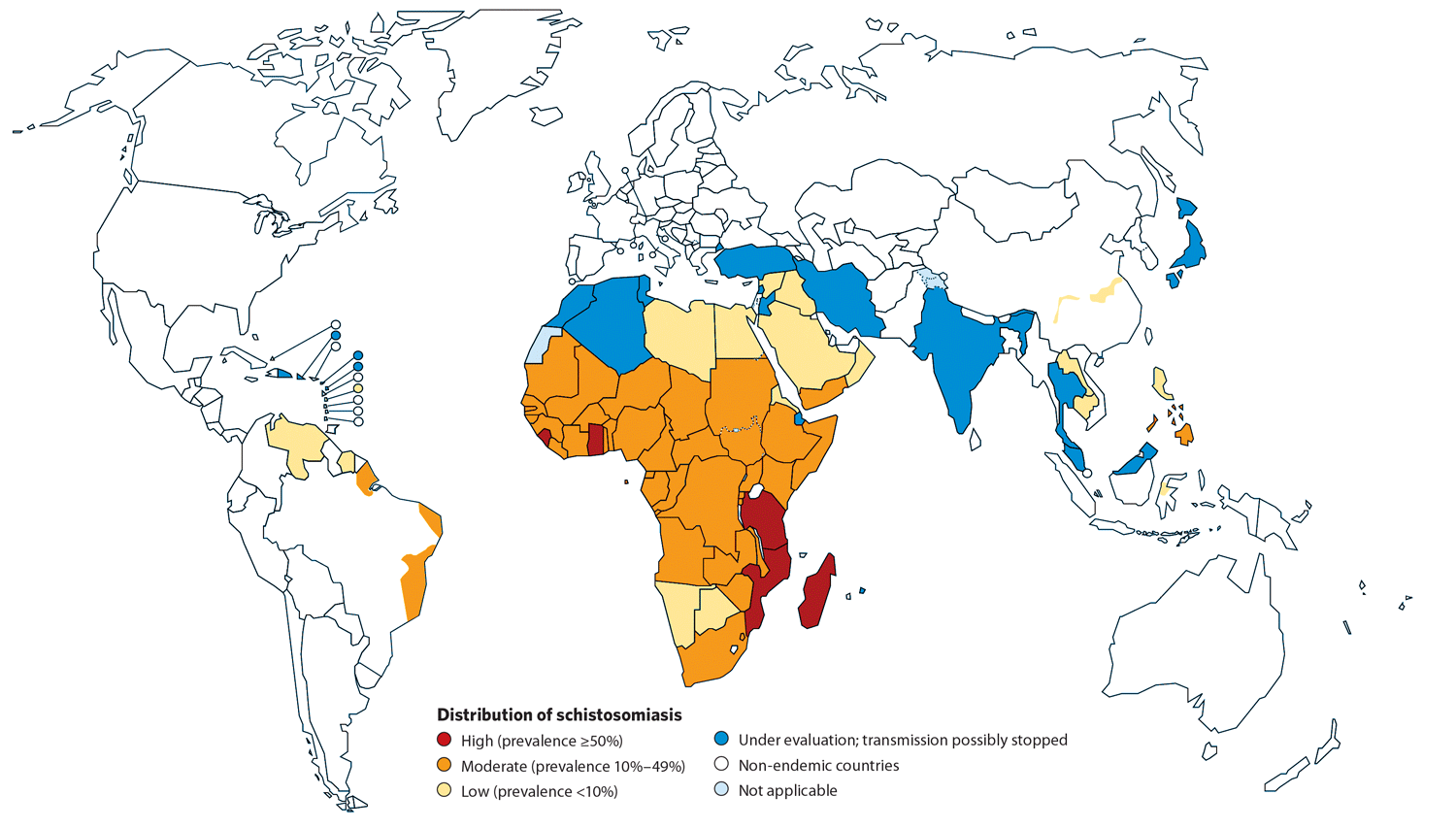
Source: World Health Organization
[/bgsection]
Infections result from contact with infested water during activities such as fishing, swimming and washing clothes.
“I’ve seen people who frequent the contaminated waters every day of their lives and are constantly exposed to the parasite,” Dr. LoVerde said.
Infections usually affect the urinary tract, liver and intestines, causing symptoms such as blood in the urine. While the disease doesn’t kill most victims, it is a chronic drain, causing subtle effects such as underperformance in schoolchildren who are anemic from blood loss. Children are stunted in their growth patterns if they have a heavy infection, Dr. LoVerde said, and their reproductive development is slower than that of uninfected children.
“It has a major impact on their future, there’s no question,” Dr. LoVerde said.
Dr. LoVerde began working in Egypt in 1970 and lived there for a year with his family, doing fieldwork on the Upper Nile near Aswan and Luxor. He also spent extensive time in Brazil.
“In the early days we searched for snails, but later we did genetic studies to learn the role people’s genes play in infection outcomes,” he said. “In Brazil we went house to house with an interpreter speaking Portuguese, asking, ‘who’s your mother, who’s your father, who’s your brother or sister, who’s your uncle, who’s your cousin?’ We used this information to establish a pedigree, and from it we found that, about 40 percent of the time, a person’s genes play a key role in infection outcomes.”
The genetic detective work also included analysis of the worms’ own genes, particularly the ones that allow the parasite to develop drug resistance. The most frequently prescribed medication, praziquantel, is plentiful and inexpensive, but as its use increases, the parasite’s resistance increases.
“Several funders are donating 250 million pills of praziquantel per year for each of the next five years to prevent the infection in Africa,” Dr. LoVerde said. “If the parasite develops drug resistance, the concern is we won’t have a backup. We need a drug that can be used in combination with praziquantel.”
The scientists are turning to a 40-year-old drug called oxamniquine for a solution. But improvements need to be made, they said. While praziquantel kills three schistosome species, oxamniquine in its original form killed only one, and that one species has been known to develop drug resistance.
In 2009, Dr. LoVerde was part of a team that published the sequence of the schistosome genome in Nature. In collaboration with geneticist Tim Anderson, Ph.D., of Texas Biomedical Research Institute, they used this information to develop a genetic map. This allowed the scientists to identify the gene responsible for drug resistance in the worms.
By 2013, working with P. John Hart, Ph.D., professor of biochemistry and director of the X-ray Crystallography Core Laboratory at the Health Science Center, the researchers determined the crystal structure of the gene’s protein—a discovery published in Science.
“We showed the details of how oxamniquine binds to this protein and how the drug works,” Dr. Hart said.
Armed with this information, the researchers turned to UTSA’s medicinal chemist Stanton McHardy, Ph.D., part of the Center for Innovative Drug Discovery, a joint initiative of the Health Science Center and UTSA. Dr. McHardy and students are taking the crystal information and making new derivatives—new modified compounds—of oxamniquine.
“Stan has made more than 80 compounds based on the information we have provided,” Dr. LoVerde said.
Two derivatives kill between 40 and 80 percent of two different schistosoma species, they found.
“It’s amazing,” Dr. LoVerde said. “What we are doing works.”
Dr. LoVerde recently traveled again to an isolated area of Brazil, where he collected samples of worms. The team found five different mutations that lead to drug resistance to oxamniquine. The team will assess ways to block the problem.
“This shows the power of genetics and molecular biology to understand and address a disease,” Dr. LoVerde said.
By understanding why the parasite becomes resistant, the team may soon have a viable option to fight the spread of schistosomiasis, and help the 261 million infected people and the half-billion more who are at risk around the world.
[bgsection pex_attr_title=”Essay: Under attack” pex_attr_subtitle=”” pex_attr_undefined=”undefined” pex_attr_style=”section-dark-bg” pex_attr_bgcolor=”f5f3e4″ pex_attr_image=”” pex_attr_imageopacity=”0.5″ pex_attr_bgimagestyle=”static” pex_attr_titlecolor=”000000″ pex_attr_textcolor=”000000″ pex_attr_height=””]By Gregory M. Anstead, M.D., Ph.D.
 In 2011, I participated in a medical mission trip to Senegal with a group from University United Methodist Church from San Antonio. In one particular village near the city of M’bour, we encountered many cases of urinary schistosomiasis, due to the parasitic flatworm Schistosoma haemotobium. In the dry season, this region of Senegal is a parched and dusty place. This village was lucky enough to have a nearby lake, which provided water for drinking and cooking, irrigation, and for raising livestock. But lurking in the water was a microscopic fork-tailed demon, the larva of the schistosoma flatworm. As the villagers gathered water from the lake, they were attacked by these larvae. The larvae burrowed into the skin of their unsuspecting victims, entered their blood stream and travelled to the liver to mature into adult flukes (flatworms). After about three weeks or so, flukes migrate to the veins of the urinary bladder to mate. The female fluke lays hundreds of eggs per day, which migrate to the urinary bladder and ureters and are excreted in the urine. If the egg-laden urine enters a water source, the life cycle of this parasite starts again.
In 2011, I participated in a medical mission trip to Senegal with a group from University United Methodist Church from San Antonio. In one particular village near the city of M’bour, we encountered many cases of urinary schistosomiasis, due to the parasitic flatworm Schistosoma haemotobium. In the dry season, this region of Senegal is a parched and dusty place. This village was lucky enough to have a nearby lake, which provided water for drinking and cooking, irrigation, and for raising livestock. But lurking in the water was a microscopic fork-tailed demon, the larva of the schistosoma flatworm. As the villagers gathered water from the lake, they were attacked by these larvae. The larvae burrowed into the skin of their unsuspecting victims, entered their blood stream and travelled to the liver to mature into adult flukes (flatworms). After about three weeks or so, flukes migrate to the veins of the urinary bladder to mate. The female fluke lays hundreds of eggs per day, which migrate to the urinary bladder and ureters and are excreted in the urine. If the egg-laden urine enters a water source, the life cycle of this parasite starts again.
In the ureters and urinary bladder, the eggs elicit an inflammatory response that leads to hematuria (blood in the urine), and blockage of the ureters, which can lead to urinary tract infections, and damage to the kidneys. With time, this prolonged inflammation can lead to bladder cancer.
In this village, we collected samples of urine for the villages and evaluated for the presence of blood with a simple dipstick test. But in many cases, a dipstick was not necessary to make a diagnosis because the urine looked almost like pure blood. Anemia is already a significant problem in the tropics because of malaria, hookworm infection, and low dietary intake of meat. Urinary schistosomiasis is yet another factor contributing to the burden of anemia in these villagers.
Fortunately, our mission team was able to provide the drug praziquantel to the infected villagers. For about $10, you could treat a case of schistosomiasis. Of course, they would inevitably get infected again in the future, but for a time, you could arrest the parasite destroying their urinary tract and stop the perpetuation of the infection in the village by eliminating the excretion of the eggs. We were gratified to help the people of one small village overcome one of their many hardships. But over 700 million people live in areas of the globe that are at risk for contracting schistosomiasis. Mass treatment programs, access to safe water, and research into more effective drugs and vaccines are needed to curb the ravages of this parasite on the impoverished peoples of developing countries.
About the author:
Dr. Gregory M. Anstead is an infectious diseases specialist with appointments at the Health Science Center and the South Texas Veterans Healthcare System. Dr. Anstead has been the director and lead clinician of the Infectious Diseases and Immunosuppression Clinics of the South Texas Veterans Health Care System. He has also treated HIV and infectious diseases patients at the Family Focused AIDS Clinical Treatment Services clinic of University Health System since 2000, and has been the clinic’s assistant director since 2011.
[/bgsection]
